In this paper, the triple differentiation of mesenchymal stem cells (MSC) and its culture Protocol are summarized.
Mesenchymal Stem Cell (MSC) have unique biological characteristics and therapeutic potential, and are becoming a "new star" in the field of disease research, and the research enthusiasm continues unabated. MSC involves many related researches, such as multiple sclerosis, arthritis, neuropathic pain, intestinal diseases, etc., and is a research hotspot in the field of transplantation and autoimmune diseases.
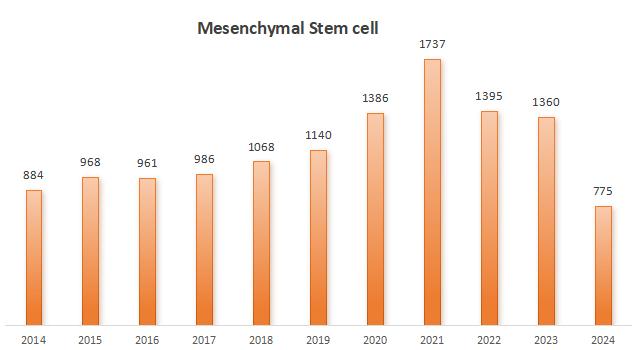
Statistics on the number of published papers in MSC field from 2014 to 2024, with data from Pubmed.
Mesenchymal stem cells (MSC) are pluripotent stem cells derived from mesoderm with high self-renewal and multi-lineage differentiation potential. They are widely distributed in various tissues of the whole body, with the advantages of easy separation and easy acquisition, and can be differentiated and expanded in vitro.
MSC is an excellent tool for tumor therapy and gene transfer research, functional genomics, drug screening, Qualcomm screening and toxicology. International Association for Cell Therapy (ISCT) defines human MSC as:
Under standard culture conditions, it is adherent growth;
CD105, CD73 and CD90 should be expressed, but CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface markers should not be expressed.
It can differentiate into osteoblasts, chondrocytes and adipocytes in vitro.
Mesenchymal stem cells come from a variety of cells or tissues, such as bone marrow, adipose tissue, peripheral blood, placental tissue, dental pulp and so on. Similarly, MSC can also differentiate into many cell types, such as osteoblasts, chondrocytes, fat cells, muscle cells, nerve cells, stromal cells and so on.
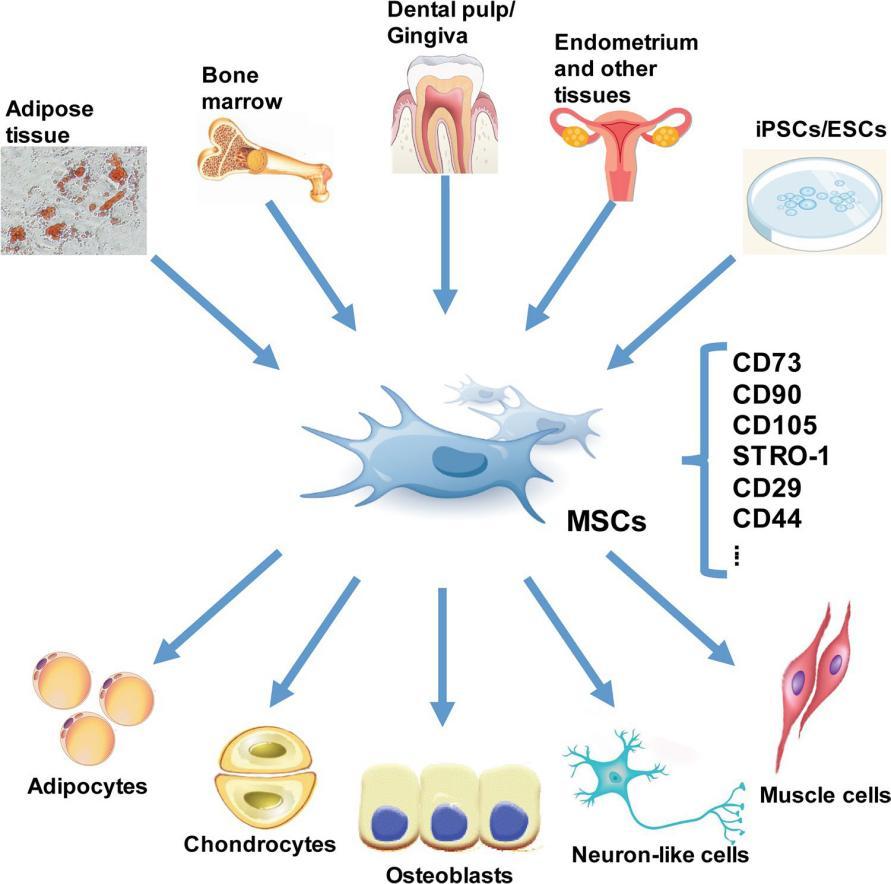
The origin and differentiation of MSC (from references)
It is worth noting that the differentiation potential of mesenchymal stem cells may vary with the source of stem cells, expansion conditions and their culture microenvironment. Three-line differentiation is the focus of MSC culture and amplification. Triple differentiation usually refers to osteogenesis, adipogenesis and chondrogenesis. In order to better understand the triple differentiation of MSC, this paper preliminarily summarizes the general process of triple differentiation of MSC and the identification methods corresponding to some markers.
Osteogenic differentiation
MSC can directionally differentiate into osteoblasts under the stimulation of various cytokines and other physical and chemical environments. Then the osteoblasts further differentiated into osteoblast precursor cells and entered a rapid proliferation period. Osteoblasts synthesize and secrete organic matrix at this stage, forming osteoid (mainly type I collagen), characterized by alkaline phosphatase, which is an early sign of osteoblast differentiation and maturity; Alkaline phosphatase appears blue under the microscope after fixation with blue, while undifferentiated cells have no obvious color.
Mature osteoblasts express ECM calcification-related proteins. Long-term osteogenic induction causes calcium ions to precipitate in the form of calcium salts, forming "bone nodules". Bone nodules can be dyed deep red by alizarin red (alizarin red reacts with calcium to produce a deep red compound), and the strength of osteogenic differentiation can be expressed by the area and depth of the pigment.
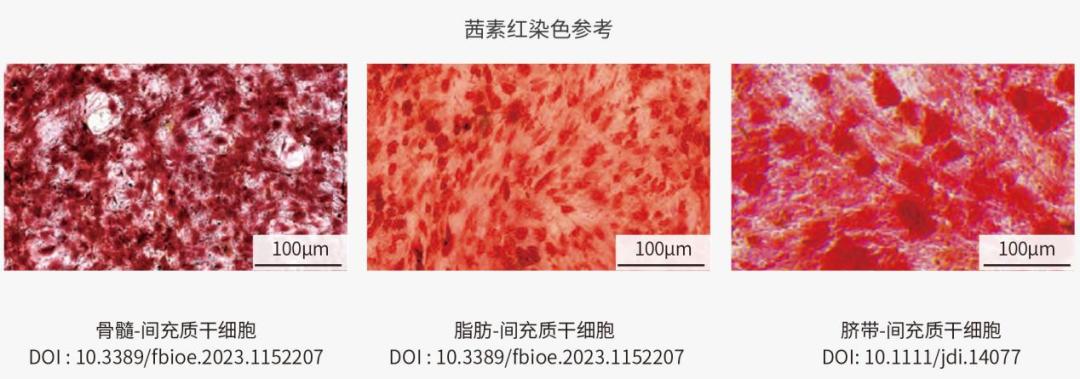
Identification of osteogenic differentiation of MSC from different sources
Chondrogenic differentiation
Firstly, MSC gathers into a cluster, and the middle cells in the cluster are transformed into a large and round cell, that is, chondrocyte, through division and differentiation. Chondrocytes produce matrix and fibers (mainly type II collagen), and the expression of type II collagen was observed by immunohistochemistry.
Generally, the differentiation results of chondrocytes were identified by Alyssin Blue. Alisine blue is an alkaline dye, which can combine with nucleic acid in cells to make the nucleus dark blue. At the same time, alisin blue combined with polysaccharides in cartilage matrix, making cartilage cells and matrix light blue.
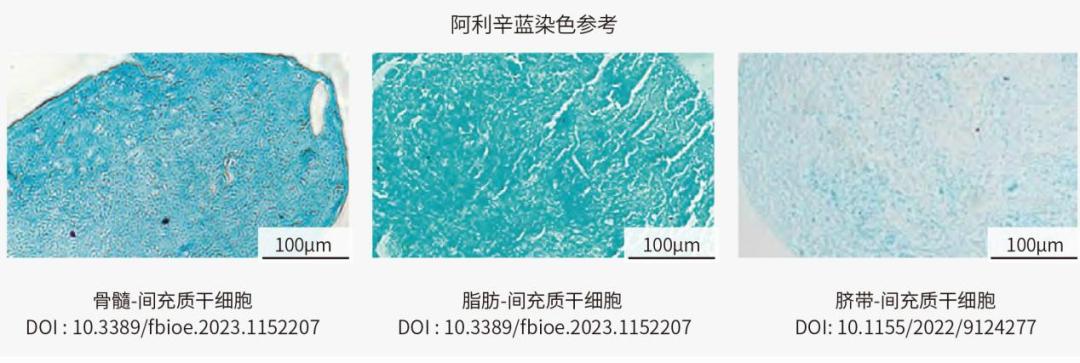
Identification of chondrogenic differentiation of MSC from different sources
Adipogenic differentiation
There are two stages of adipogenic differentiation of MSC: first, it differentiates into adipose precursor cells, and then it differentiates into adipocytes under the influence of specific stimuli (such as C/EBPs and PPAR-γ).
Cells keep accumulating fat droplets in the cytoplasm and getting bigger. After dyeing with oil red O, it is red under the microscope. Undifferentiated cells have no obvious color.
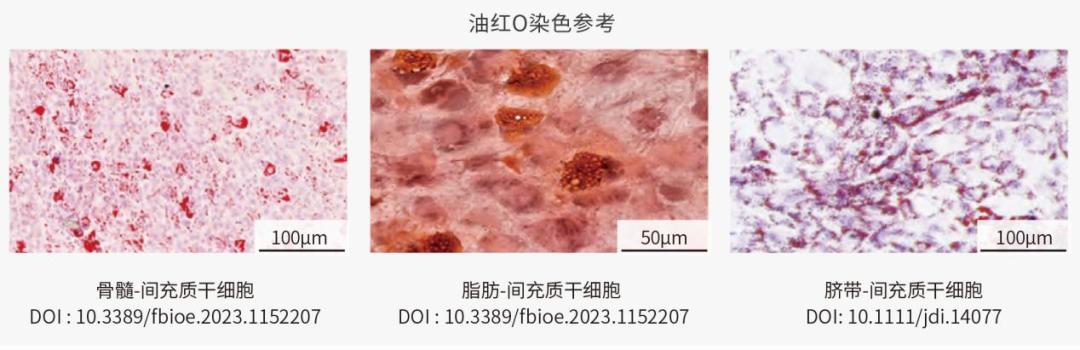
Identification of chondrogenic differentiation of MSC from different sources
Various factors will affect the differentiation potential. Such as culture environment, growth factors and the existence of other cells, will affect the differentiation process. Specific signal molecules, such as bone morphogenetic protein (BMP) or Wingless related integration site (Wnt), can promote the differentiation of stem cells into bone cells or nerve cells, respectively.
The research on mesenchymal stem cells is so hot that Yiqiao Shenzhou has compiled a detailed MSC operation guide. Protocol is full of dry goods, in addition to the above three-line differentiation identification examples, it also includes how to isolate mesenchymal stem cells, how to subculture and expand them, and how to determine that the expanded cells are mesenchymal stem cells. Welcome to download and learn!
Click on the link above to download the Protocol.
References:
Dragomirka Jovic. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Reviews and Reports, 2022. https://doi.org/10.1007/s12015-022-10369-1
Xing Liang Fan, et al. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cellular and Molecular Life Sciences, 2020. https://doi.org/10.1007/s00018-020-03454-6
Yu Han, et al. Mesenchymal Stem Cells for Regenerative Medicine. Cells, 2019. doi:10.3390/cells8080886
Tilotta V, et al. Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro. Front. Bioeng. Biotechnol. 2023. doi: 10.3389/fbioe.2023.1152207

Read the original text