Helicobacter pylori vaccine, how far is it from us?
Original porridge medical digestive liver disease channel included in the topic #2020 CGC18.
I’ve heard of many kinds of vaccines. Is there an Hp vaccine?
Speaking of Helicobacter pylori (Hp), I think everyone is familiar with it. Hp is a gram-negative spiral bacteria, which mainly spreads from person to person through oral route [1]. The infection rate of Hp in Chinese is very high, exceeding 50%, and the current research proves that Hp has an ambiguous relationship with gastric cancer, and the World Health Organization (WHO) has identified it as a grade I carcinogen of gastric cancer.
Traditional treatment has always relied mainly on acid inhibitors combined with antibiotics to eliminate Hp to treat related diseases caused by it, but the abuse of antibiotics has led to the rising drug resistance rate of Hp. So, can we eradicate Hp? I’ve heard of many kinds of vaccines. Is there an Hp vaccine?
At the just-concluded 20th National Academic Conference on Digestive Diseases of Chinese Medical Association, Professor Zou Quanming from the Biopharmaceutical Teaching and Research Section of the Department of Pharmacy of Army Military Medical University gave a wonderful report on the theme "How far is Hp vaccine from us?".
Do we need Hp vaccine?
First of all, Professor Zou Quanming raised a question: Do we need Hp vaccine?
▎Hp is closely related to gastric cancer and has serious harm.
At present, there are three different views on Hp in the medical field:
1. The pathogenicity of Hp is serious, and vaccine is needed for radical cure;
2. Hp is still helpful to the human body, even probiotics;
3. Hp and human beings have coexisted for a long time, and their existence is reasonable.
These different viewpoints have seriously affected the research and appearance of Hp vaccine.
According to the data released by the National Cancer Center in 2017, there are about 680,000 new cases of gastric cancer in China each year, accounting for nearly one-third of all cancers in China, accounting for about 50% of the total incidence in the world, and the mortality rate also accounts for about 50% of the world total. Hp infection can almost always cause active inflammation of gastric mucosa. On the basis of chronic inflammation, some patients can also develop a series of diseases such as peptic ulcer and gastric cancer (1%).
Hp infection has brought great disease burden and economic loss to people, but at present, people pay insufficient attention to Hp. Professor Zou Quanming emphasized that Hp is very harmful and we need Hp vaccine.
▎ At present, antibiotics are used to treat Hp, but the symptoms are not cured.
Traditional treatment has always relied mainly on acid inhibitors combined with antibiotics to eliminate Hp to treat related diseases, but antibiotics can only eliminate the existence of individual Hp or suppress its proliferation, and can not achieve the goal of eradicating Hp for the whole society. And the drug resistance caused by frequent use of antibiotics, such as clarithromycin and metronidazole, has become a serious problem in some countries. In addition, the use of antibacterial therapy to eradicate Hp is not sustainable. In some countries, the annual recurrence rate of Hp is as high as 15%-30%.
Professor Zou Quanming said that China, Japan, South Korea and other countries in the Far East, including many countries in Eastern Europe, have a high incidence of gastric cancer. Under the condition of extensive vaccination, even if the hidden Hp infection of patients is not detected, the incidence of gastric cancer can be reduced. In developed countries, it is calculated that a 10-year vaccination program can greatly reduce the prevalence of ulcer and gastric cancer caused by Hp, and also reduce the incidence of related diseases and economic losses.
Basic questions to be answered in Hp vaccine research
However, the research of Hp vaccine is not smooth sailing. Professor Zou Quanming pointed out that it is very difficult to study Hp vaccine and the following problems need to be solved:
1. Why is the immune response caused by natural infection of the host not protective?
2. What are the mechanisms and differences between immune protective response and pathological injury?
3. Screening and transformation of vaccine effective antigen?
In addition, the immune mechanism of Hp infection is very complicated.
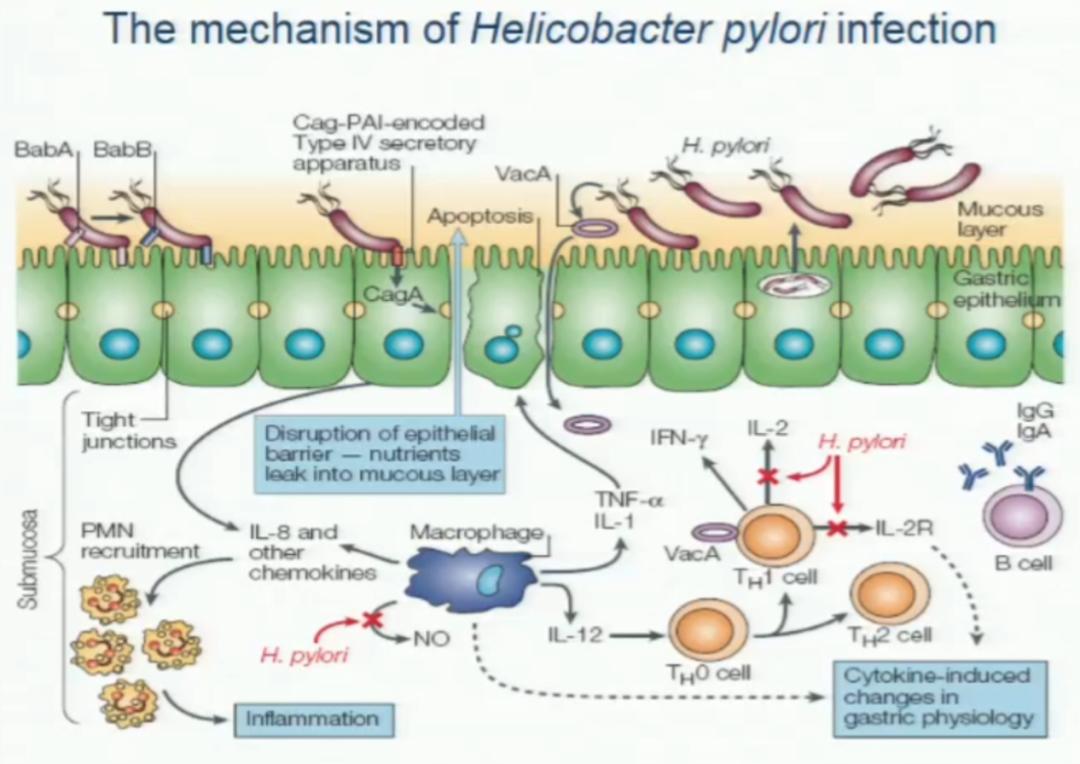
Figure 1: Infection immune mechanism of 1:Hp
Research progress of preventive Hp vaccine
Next, Professor Zou Quanming introduced the research progress of Hp vaccine at home and abroad:
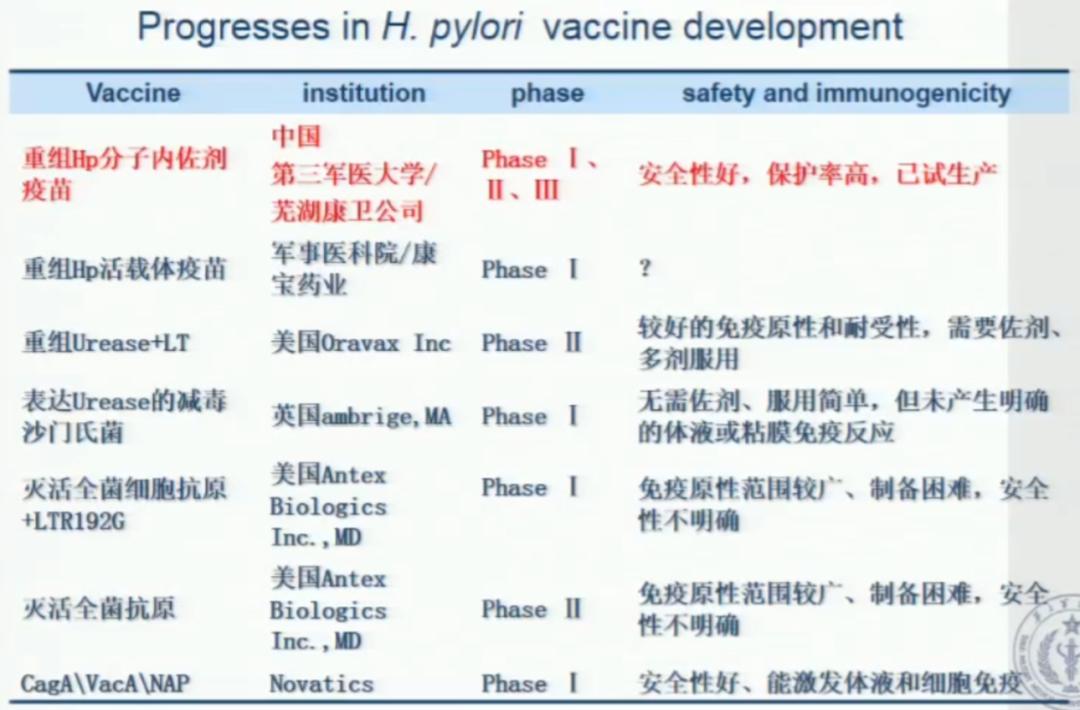
Figure 2: Research progress of Hp vaccine at home and abroad.
Professor Zou Quanming and his research team established the strategy of "intramolecular adjuvant mucosal vaccine of Hp", which has been verified by practice. This technique can produce sIgA antibody in gastrointestinal mucus (which is completely different from serum IgG antibody), thus blocking Hp infection.

Fig. 3: Mechanism of action of intramolecular adjuvant mucosal vaccine of 3:Hp.
Animal experiments show that the protective rate of the oral recombinant Hp vaccine in mice, rats and rhesus monkeys is more than 80%.
In addition, Professor Zou Quanming and his research team established an efficient system for screening components of Hp vaccine for the first time, and successfully constructed an engineering strain of Hp vaccine that can be used for production. More than 300 strains of Hp and more than 1,500 candidate protein components were collected from patients in 20 provinces and cities in China, and finally the Hp vaccine components were selected, and an efficient and stable vaccine purification process was established. The purity of the produced vaccine protein was over 80%, and the output was 400,000 mg/time (reaching the production scale).
In 2015, the clinical research results of "Oral Helicobacter pylori vaccine phase III" conducted by Professor Zou Quanming and his research team were published online in the top medical journal The Lancet.

Figure 4: The research results are published online in The Lancet.
In this study, more than 30 technical specifications, standards and experimental detection technologies necessary for human clinical trials of Hp vaccine I, II and III were established. And through the invention and creation of the above key technologies, more than 4,000 volunteers aged 6-15 have been developed and completed in phase I, II and III human clinical trials.
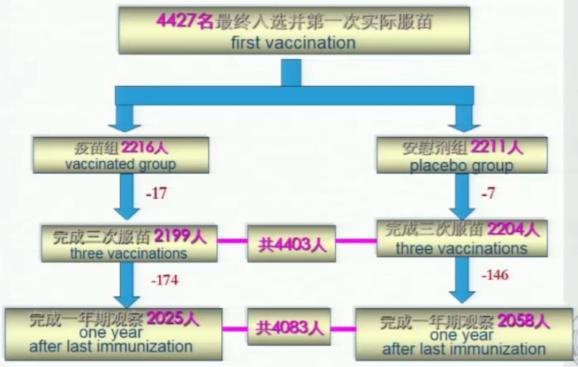
Figure 5: Distribution and shedding of subjects
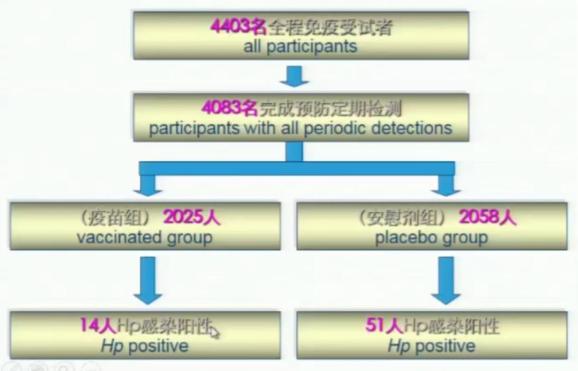
Figure 6: Analysis results of infection prevention effect
The results showed that there were 64 cases of Hp infection during the follow-up period, that is, 14 cases were infected in the vaccine group (2025 people) and 51 cases were infected in the control group (2058 people). The total protection rate of oral recombinant Hp vaccine against infection was 71.8%.
In terms of safety, 157 (7%) people in the experimental group and 161 (7%) people in the control group had one or more adverse reactions during the study. Among them, 5 (< 1%) people in the experimental group and 7 (< 1%) people in the control group had serious adverse reactions, but they all thought it had nothing to do with vaccination [2].
This shows that oral recombinant Hp vaccine is "effective, safe and immunogenic" among children who are not infected with Hp, which can significantly reduce the incidence of Hp infection.
Professor Zou Quanming said that he also took the Hp vaccine at that time. Up to now, he has been tested for Hp once a year, and the results have been negative.
This study is the first phase III trial of Hp vaccine in the world. Professor Philip Sutton of Melbourne University in Australia made a special comment on this study: "The successful development of this vaccine has taken an important step towards preventing gastric cancer caused by Hp. This will rekindle human passion for this important topic, further encourage more investment, and promote continuous progress in this field through in-depth clinical trials. "
The Hp vaccine is still in the approval stage and has not been approved for marketing. At the scene, Professor Zou Quanming also expressed his expectation and called on academic organizations and Hp study groups to jointly promote the listing of Hp vaccine.
Reference source:
[1] Chinese Medical Association, et al. Guidelines for primary diagnosis and treatment of Helicobacter pylori infection (2019). Chinese Journal of General Practitioners .2020,5 (19) 5.
[2]Ming Zeng,Prof Xu-Hu Mao,et,al.Efficacy, Safety, and Immunogenicity of an Oral Recombinant Helicobacter pylori Vaccine in Children in China: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial.The Lancet.2015,6(30).
Review of this article

Professor Cao xiaocang
Chief physician, professor, master tutor of Tianjin Medical University General Hospital of Gastroenterology.
Member of Digestive Endoscopy Committee of Inflammatory Bowel Disease Group of Digestive Branch of Chinese Medical Association.
Vice Chairman of the Youth Committee of Behavioral Medicine Branch of Chinese Medical Association
Member of Clinical Epidemiology Collaboration Group of Digestive Branch of Chinese Medical Association.
Member of Inflammatory Bowel Disease Group, Committee of Gastroenterology Branch of China Medical Equipment Association.
Member of anorectal difficult disease Committee of inflammatory bowel disease Committee of anorectal physician branch of Chinese Medical Doctor Association
Member of Expert Committee of Inflammatory Bowel Disease, Gastroenterology Professional Committee, China Society of Integrated Traditional Chinese and Western Medicine.
Member of the Expert Committee of Inflammatory Bowel Disease of Beijing Medical Award Foundation.
Standing Committee of Inflammatory Bowel Disease Alliance of Wu Jieping Medical Foundation, Standing Committee of Intestinal Microecology Professional Committee
Standing Committee of the Committee of Stem Cell Engineering Technology Branch of China Biomedical Engineering Society
Vice Chairman of Inflammatory Bowel Disease Group of Gastroenterology Branch of Tianjin Medical Association
This article starts: medical digestive liver disease channel
Author: CGC report group porridge
Original title: Helicobacter pylori vaccine, how far is it from us? 》
Read the original text